| |
| ≡ Short Path Evaporator |
>>>>Present page :Short Path Evaporator |
|
|
|
 The
difference of pressing boiling membrane and condensation
is the driving force that the steam flows into,
will cause the flow of the steam to the small
pressure when falling. Is it require in surface
and short distance very the condensation of seething
with excitement to operate under 1mbar, the distiller
made on the basis of this principle is called
the short distance distiller . Short distance
distiller (molecule distillation ) have one built-in
condenser in heat opposite the Taxi, enable operating
the pressure and drop to 0. 001mbar。 The
difference of pressing boiling membrane and condensation
is the driving force that the steam flows into,
will cause the flow of the steam to the small
pressure when falling. Is it require in surface
and short distance very the condensation of seething
with excitement to operate under 1mbar, the distiller
made on the basis of this principle is called
the short distance distiller . Short distance
distiller (molecule distillation ) have one built-in
condenser in heat opposite the Taxi, enable operating
the pressure and drop to 0. 001mbar。
The short distance distiller is a job in 1.
001mbar hot to separate technological course under
the pressure, it low boiling temperature, suit
temperature sensing, high boiling point thing
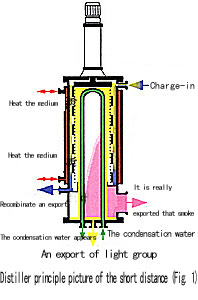
very much. It is formed basically: Heat the cylinder
type barrel which inserts set , the rotor and
built-in condenser ; Equipped with the membrane
shaving device and defended splashing the device
accurately on the mounting bracket of the rotor
. The built-in condenser lies in the centre of
the evaporimeter , the rotor is rotated between
cylinder type barrel and condenser。
Short distance distiller by which heat outside
vertical round barrel, lie in centre condenser
of it and whirling membrane blowing device make
up between distiller and condenser 。
The distillation course is: Supplies join from
top of evaporimeter, distribute their in heating
the surface evenly in succession by material liquid
distributing device of rotor, shaved the membrane
device and shaved the material liquid into the
liquid film with extremely thin taking the form
of turbulance one storey immediately , and move
forward downwards with the spiral form . In this
course , from heating the light molecule that
the ease produces on the surface , become liquid
through the short condensation on the built-in
condenser of route and nearly getting without
colliding , and is in charge of the condenser
and flow, through lying in producing material
and is in charge of discharging of the evaporimeter
bottom; Incomplete liquid namely heavy member
collects in the round passway under the district
of heating, and then flow out while managing through
producing material of the side 。
The short distance distiller is still suited
to carrying on the molecule distillation. The
molecule flows from heating the surface to the
condenser surface directly. As drawn, the molecule
distillation course can take place four steps
as follows :
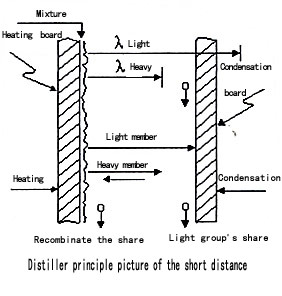 |
1、 Whether molecule subject to evaporate
surface spread from liquid phase. Usually,
the rate of diffusion that the liquid takes
a fancy to is a main factor of controlling
the molecule distillation tempo, so should
try one's best to reduce one layer of thickness
of thin liquid and strengthen the flow on
storey of the liquid 。
2、 One floor of freedom on the surface
in liquid evaporates in the molecule: The
evaporation rate rises with rising of temperature,
but separate the factor to reduce with rising
of temperature sometimes, so should in order
to process hot stability of material as
prerequisite , choose economy reasonable
distillation temperature 。
3、 The molecule is from evaporating the
surface to fly to penetrate the condensation:
The vapour molecule flies in the course
penetrated the condensation of facing of
evaporation, perhaps collide each other
each other, may collide with the remaining
air and molecule between the two sides too.
Because it is far heavy in the air and molecule
to evaporate molecules , and mostly have
the same movement directions, so their one's
own collision penetrates the direction and
evaporation rate influence not big in flying.
And residual gas molecule disorderly and
unsystematic hot motion state between two
sides, so residual gas amount, molecule
of figure to is it is it penetrate direction
and main factor of evaporation rate to fly
to influence 。
4、 Condensation the condensation of molecule:
Guarantee cold and hot two sides have enough
temperature getting bad , form , condensation
of surface reasonable smooth to think condensation
step can in finish in the twinkling of an
eye, so the form of choosing rational condenser
is quite important 。 |
Therefore can have , the condition of the molecule
distillation is :
1、 It is must the low very in partial pressure
of gas to be remaining,it make length Cheng not
free not average of gas not remaining for distiller
and condenser surface from multiple 。
2、 Under the saturation pressure, the average
free Cheng's length of the steam and molecule
must have the same order of magnitude with the
distance between the evaporimeter and condenser
surface 。
Under this ideal condition here , evaporate
and happen from remaining gas and molecule in
a situation that there is not any obstacle. All
steam molecules reach the condenser surface in
the course of running into other members and returning
to liquidly . The evaporation rate reaches the
possible maximum under the temperature that is
in. The evaporation rate is in direct proportion
to pressure, therefore, the liquid amount has
appeared relatively smaller in the heating up
in a steamer of molecule distillation 。
Among SPE short distance distillation, condenser
and distance to heat surface about 200mm, remaining
pressure of gas at the 10-3mbar, remaining gas
molecule free length Cheng about 2 times long
on average. The short distance distiller can totally
meet all essential conditions of molecule distillation
。
The molecule distillation has the following
characteristics :
1、 The ordinary distillation is separated under
boiling temperature, the molecule distillation
can go on under any temperature , so long as there
is bad temperature between cold and hot two sides
, can reach the item of separating to click 。
2、 Ordinary distillation to evaporate reversible
course in condensation, liquid phase and angry
to can form balanced state while being alternate;
And molecule distillation among the course, from
evaporate surface molecule that ease produce is
it penetrate on condensation to fly directly,
do not collide with other members in the middle
, has not returned and evaporated Taxi possibility
in theory , so, the molecule distillation course
is irreversible 。
3、 The ordinary distillation has the drum to
steep , seethe with excitement the phenomenon;
The molecule distillation course is liquid one
layer of freedom on the surface is evaporated,
there is not the drum that steep the phenomenon
。
4、 Show that it relates to the fact that the
steam of yuan presses the proportion in the group
to separate the separation factor of ability in
ordinary distillation, show that it relates to
the fact that the steam of yuan presses with the
proportion of molecular weight in the group to
separate the separation factor of ability in molecule
distillation, and can be asked out by the relative
evaporation rate 。
|
|
|
|
|